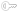invivodata First to Deploy the Tungsten E2 in Clinical Trials
invivodata, an industry leader in electronic patient reported outcome (PRO) solutions for clinical research, has plans to deploy between 5,000 and 7,000 Tungsten E2 handhelds from palmOne for use in worldwide clinical trials during the next 90 days.
As the demand for invivodata's unique electronic diary system, DiaryPRO continues to grow, the number of deployed devices could easily be four times that amount by the end of 2005. By combining behavioral science and clinical expertise with palmOne's proven handheld technology, invivodata's electronic diary system increases patient compliance with reporting requirements to more than 90 percent, up from less than 20 percent with conventional paper-based diaries. This dramatic increase in compliance delivers higher-integrity PRO data that trial sponsors can rely upon as they evaluate the effectiveness of new treatments. In fact, invivodata's electronic diary has successfully delivered key data for multiple new drug approvals granted by the Food and Drug Administration (FDA).
 "By deploying our scientific expertise on palmOne's newest handheld product, we will continue to deliver unique solutions that streamline the clinical drug development process and put patients back at the center of research where they belong," said Doug Engfer, president and chief executive officer of invivodata. "We look forward to standardizing on the Tungsten E2 as our device of choice and leveraging its flexibility and ease of use in clinical trials throughout the world."
"By deploying our scientific expertise on palmOne's newest handheld product, we will continue to deliver unique solutions that streamline the clinical drug development process and put patients back at the center of research where they belong," said Doug Engfer, president and chief executive officer of invivodata. "We look forward to standardizing on the Tungsten E2 as our device of choice and leveraging its flexibility and ease of use in clinical trials throughout the world."
The Tungsten E2 builds on the popularity of the Tungsten E handheld, which has sold nearly 2 million units worldwide. The Tungsten E2's non-volatile memory and Bluetooth technology are key enablers for clinical trial use. While non-volatile memory keeps trial data secure, Bluetooth technology enables integration with physiological devices, such as peak flow meters, a standard tool used in respiratory trials.
"With the ever-increasing importance being placed on high-quality patient- reported data, we answered our customers' demand by delivering a handheld computer with non-volatile, flash memory to keep all information on the product safe, even if there's no time to recharge," said Tara Griffin, vice president, Americas Sales, for palmOne. "It's rewarding to see how invivodata and its pharmaceutical, biotech and medical device company clients will benefit from all of the Tungsten E2's new features."
Leave a comment...
You must be registered and logged in to add comments.
Latest Comments
- I got one -Tuckermaclain
- RE: Don't we have this already? -Tuckermaclain
- RE: Palm brand will return in 2018, with devices built by TCL -richf
- RE: Palm brand will return in 2018, with devices built by TCL -dmitrygr
- Palm phone on HDblog -palmato
- Palm PVG100 -hgoldner
- RE: Like Deja Vu -PacManFoo
- Like Deja Vu -T_W